1. Liver development
In the liver, hepatocytes, biliary epithelial cells (BECs), and sinusoid endothelial cells (SECs) form hepatic cords, intrahepatic bile ducts (IHBD), and sinusoids, respectively. These three dimensional (3D) tissue structures are important for physiological functions of the liver. We have been investigating 3D morphogenesis of the liver tissue structures during development and regeneration (References;1,2).
IHBD is a tubular structure consisting of BECs, which drains bile produced by hepatocytes into the duodenum. The IHBD network consists of large ducts running along portal veins (PVs) and small ductules forming a mesh-like network around PVs. This mature “hierarchical network” is established by 1 week after birth. The continuous luminal network is already evident at embryonic day 17 (E17). The fine homogenous network is reorganized into the mature hierarchical network between E17 and E18 (Fig.1). During this period, bile canaliculi, the fine apical luminal network of hepatocytes, rapidly extend. We can nakedly observe that the color of the intestine turns to yellow atE18. These results suggest that the influx of bile including bilirubin into IHBDs, rapidly increases between E17 and E18 (Fig. 2). Expression of Ugt1a1, a crucial enzyme for direct bilirubin formation, is up-regulated in E18 liver compared to E17. Continuity of BCs in E17 livers is often interrupted and the average length of each BC was shorter than in later stages. When the formation of bile canaliculi was blocked between E16 and E18 by a MRP2 inhibitor, benzbromarone, the structural rearrangement of IHBDs is significantly suppressed (Fig.3). Therefore, the increased influx of bile juice into IHBDs likely promotes this dynamic structural transition (1,3).
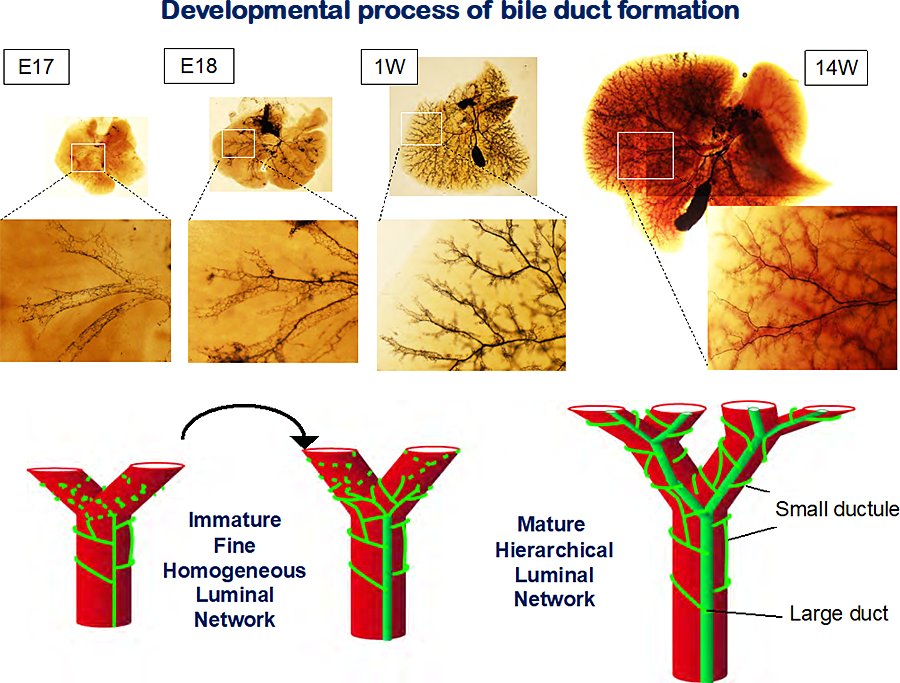 Figure 1 Development of intrahepatic bile ducts.
Figure 1 Development of intrahepatic bile ducts.
In fetal liver, IHBDs consist of fine homogeneous tubules, whereas IHBDs consist of larges ducts and fine tubules in adult livers. The structure of IHBDs transfer from immature to mature luminal network between E17 and E18. Carbon ink was injected from the junction between common bile ducts and the duodenum. The liver tissue was cleared in BABB.
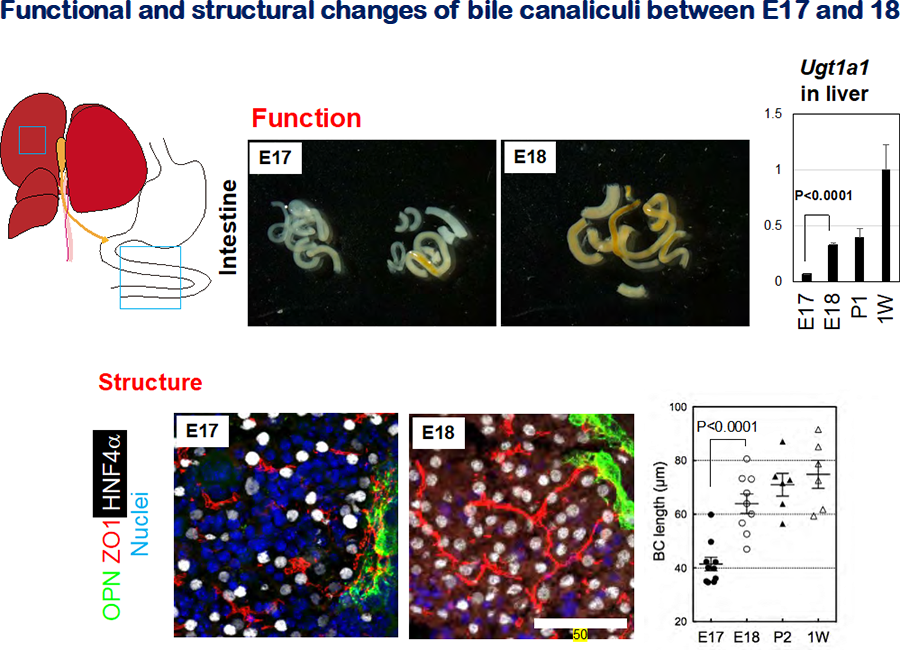 Figure 2 Formation of the bile canalicular network during liver development
Figure 2 Formation of the bile canalicular network during liver development
- The appearance of E17 and E18 intestine. The color of E17 intestine is mostly white, whereas that of E18 intestine is totally yellow. This color change reflects the influx of bile containing bilirubin into the intestine.
- Expression of Ugt1a1 in late fetal and neonatal livers. Ugt1a1 , an enzyme glucuronidating bilirubin, is significantly upregulated between E17 and E18.
- Bile canalicular structures in E17 and E18 liver. ZO1+ bile canalicular structures are discontinuous in E17 liver, whereas they are organized into the continuous network is established in E18 liver. Liver sections were stained with anti-ZO1 to visualize BC structures. The sections were also stained with anti-OPN and anti-HNF4α to reveal cholangiocytes and hepatocytes, respectively.
- BC length in late fetal and postnatal livers. BC lengths increase during development, most rapidly between E17 and E18. BC length was deduced by measuring the length of ZO1+ membrane structures.
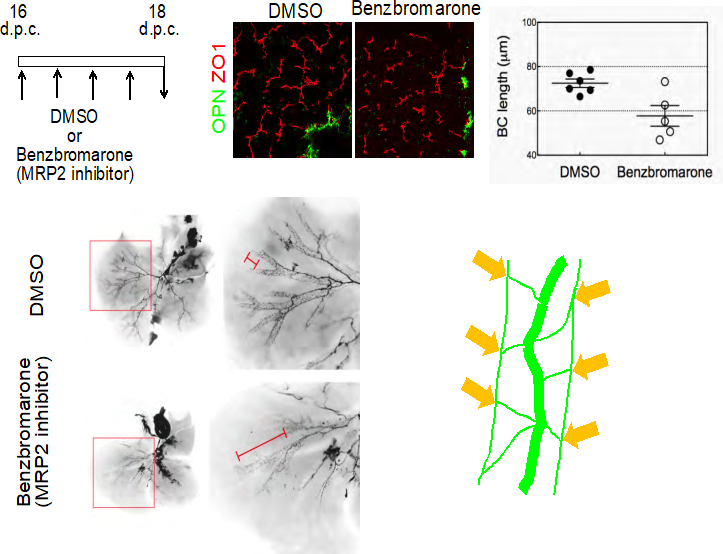 Fig. 3 Formation of the bile canalicular network induces dramatic remodeling of IHBDs.
Fig. 3 Formation of the bile canalicular network induces dramatic remodeling of IHBDs.
- Inhibition of MRP2 blocks formation of BCs. Benzbromarone, a MRP2 inhibitor, blocks formation of BCs (arrowheads). Benzbromarone (750 nmol/g) was intraperitoneally injected into pregnant mice every 12 h between 16 and 18 d.p.c. The length of BC structures in embryonic liver is reduced by benzbromarone.
- Blocking BC formation suppresses the structural rearrangement of IHBDs. In controls, most IHBDs are organized in the hierarchical network (panels 1 & 2), whereas IHBDs remaining in the homogenous network are evident in the benzbromarone-treated liver (panels 3 & 4). The lengths of the homogenous biliary network IHBDs were measured after ink injection in E18 embryos.
In mouse livers, nerve fibers are localized within the portal triads. The intrahepatic nerve network has been implicated in regulation of metabolisms, bile secretion, and blood flow. Nerve fibers start invading into the liver tissue in the perinatal period and the whole liver tissue is innervated by 3 weeks after birth. Nerve growth factor (NGF) is secreted from cholangiocytes and portal mesenchymal cells during intrahepatic innervation (Fig. 4). When the biliary network is damaged by administration of 4-4’-diaminodiphenylmethane, the nerve network was also destructed. Along with biliary regeneration, the nerve network is reestablished depending on the NGF signal. Moreover, with ectopic expression of NGF in hepatocytes, the nerve fibers enter the liver parenchyma and extend toward the pericentral area (Fig.5). Thus, IHBDs guide the extension of nerve fibers in the liver tissue by supplying NGF during development and regeneration (2).
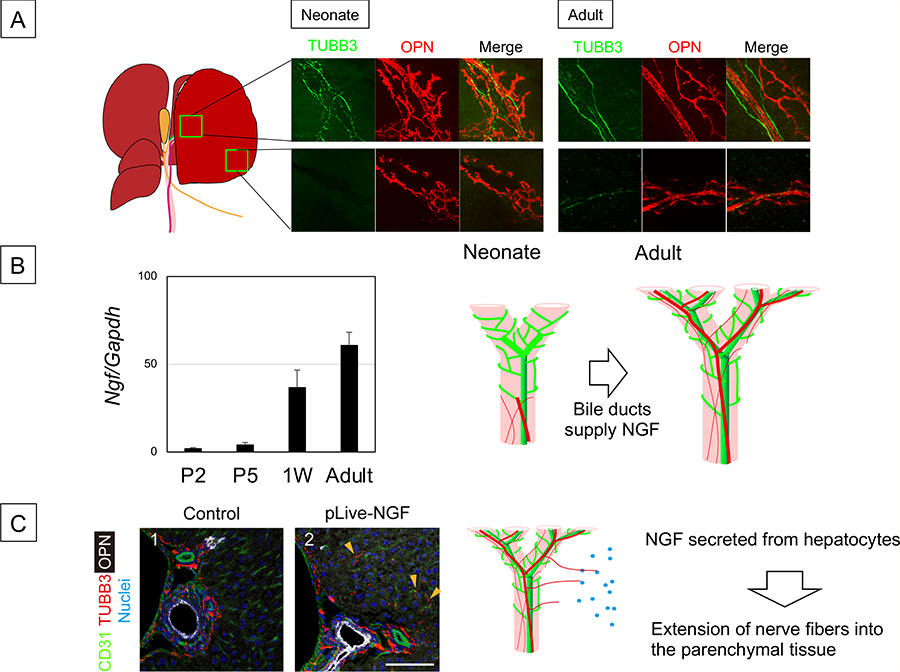 Figure 4 Development of intrahepatic nerve network
Figure 4 Development of intrahepatic nerve network
- Comparison of intrahepatic nerve network between neonate and adult liver.
- IHBDs are associated with nerve fibers in the hilum but not in the periphery of the liver at 1W after birth. On the other hand, IHBDs are thoroughly associated with nerve fibers. Biliary epithelial cells start to express Ngf at neonatal period. IHBDs form a tubular network in late fetal stage but not express Ngf. Depending on NGF derived from neonatal IHBDs, nerve fibers extend into the liver tissue.
- Ectopic expression of NGF expands the nerve network. TUBB3+ nerve fibers are extended to the liver parenchyma at 4W after administration of pLIVE-NGF, whereas they are limited near OPN+ BECs and around the PV in the control. A plasmid coding NGF (pLIVE-NGF) was introduced to MHs by hydrodynamic tail vein injection (HTVi). Liver sections were stained with anti-OPN, anti-TUBB3, and anti-HNF4α antibodies and nuclei were counterstained with Hoechst 33342.
- Tanimizu N, Kaneko K, Itoh T, Ichinohe N, Ishii M, Mizuguchi T, Hirata K, Miyajima A, Mitaka T. Intrahepatic bile ducts are developed through formation of homogeneous continuous luminal network and its dynamic rearrangement. Hepatology, 2016; 64(1):175-188
- Tanimizu N, Ichinohe N, Mitaka T. Intrahepatic bile ducts guide formation of the hepatic nervous system in developing mouse liver. Development, 2018; 145 (9): pil; dev159095
- Tanimizu N, Mitaka T. Epithelial morphogenesis during liver development. Cold Spring Harb Perspect Biol, 2017; 9(8): pii: a027862