2. Stem/progenitor cells involved in normal and pathophysiological conditions of livers
Dlk-1+ hepatocytes are bipotential liver stem/progenitor cells (LPCs) in fetal liver, whereas it has been reported that adult LPCs are enriched in cells positive for BEC markers including epithelial cell adhesion molecule (EpCAM), osteopontin, and Sry HMG box protein 9 (SOX9) (3). EpCAM+ BECs isolated from neonatal liver show bidirectional differentiation potential both in vitro and in vivo (Fig. 1). However, they lose such capability in adult liver (4).
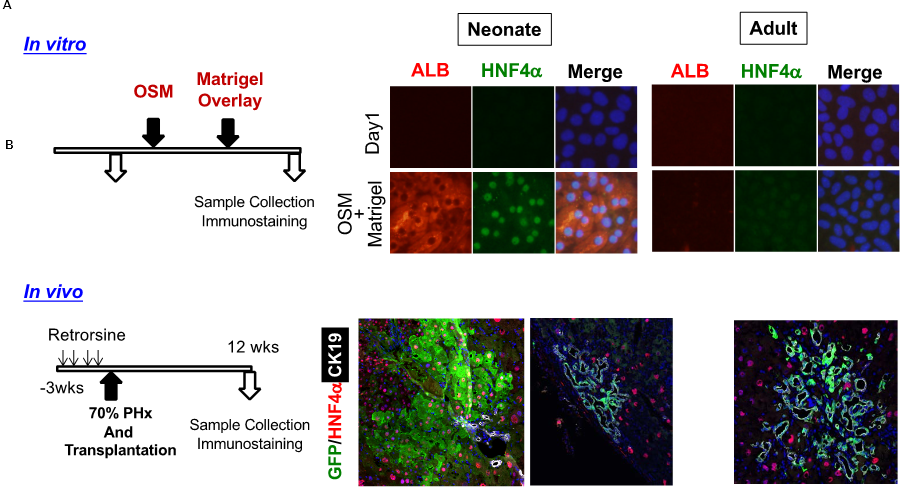 Fig. 1 Transition of differentiation potential of liver progenitor cells.
Fig. 1 Transition of differentiation potential of liver progenitor cells.
- Neonatal, but not adult, EpCAM(+) liver progenitor cells (LPCs) differentiate to ALB(+)HNF4α(+) hepatocytes in vitro.
- When transplanted to mice whose liver tissue is resected after retrorsine treatment, neonatal EpCAM(+) cells are engrafted as hepatocytes as well as biliary epithelial cells in the recipient liver . On the other hand, adult EpCAM(+) cells are engrafted only as bile duct-like structures.
On the other hand, we have demonstrated that hepatocytes with relatively small size called small hepatocytes (SH) isolated from adult liver have the colony-forming capability. Mouse SHs are enriched in ICAM-1+EpCAM– cells. ICAM-1+ hepatocytes clonally proliferate in vitro and can differentiate into mature hepatocytes (MHs) in vitro and in vivo after transplantation (Fig. 2). ICAM-1+ cells are mostly mononuclear hepatocytes and similar but not identical gene expression profile as compared with MHs (5,6). Our results suggest that biliary and hepatocyte-committed progenitors have more significant roles in healthy adult liver than putative bipotential LPCs.
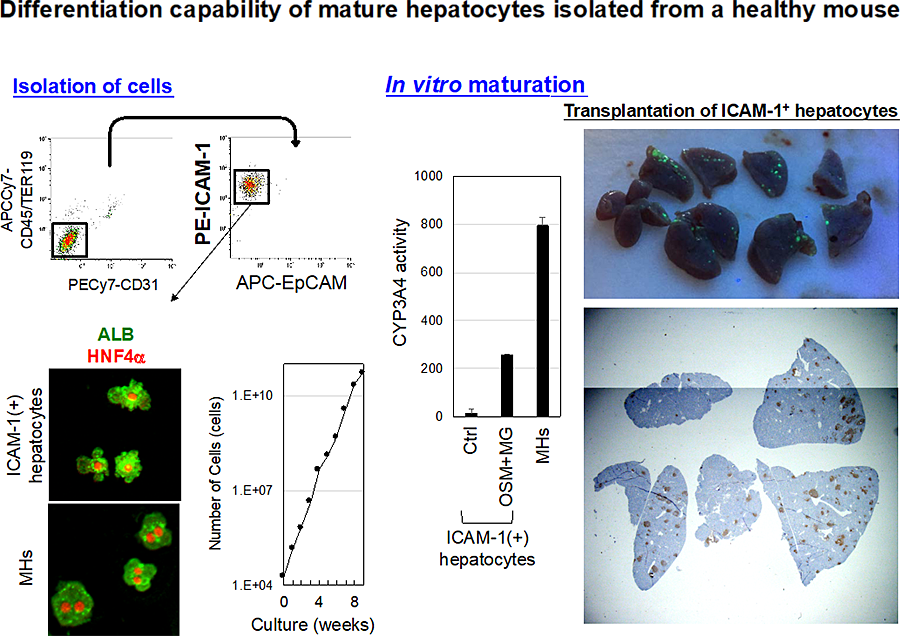 Figure 2 Mouse adult hepatocyte progenitors differentiate to functional hepatocytes both in vitro and in vivo.
Figure 2 Mouse adult hepatocyte progenitors differentiate to functional hepatocytes both in vitro and in vivo.
Adult hepatocyte progenitors are enriched in CD45(-)TER119(-)CD31(-)ICAM-1(+)EpCAM(-) fraction. ICAM-1(+) hepatocytes are relatively small cells as compared with MHs and positive for HNF4α and ALB. ICAM-1(+) hepatocytes keep their proliferative capability at least for 8 weeks and potential to differentiate to functional hepatocytes in vitro. ICAM-1(+) hepatocytes labeled with GFP are engrafted in the recipient liver.
It is well known that the liver has strong regenerative capability. After transient injuries, hepatocytes and BECs simply proliferate and compensate the lost tissue. Upon chronic injuries, the expansion of biliary structures and small hepatocyte-like progenitors are well characterized. In addition, we found that “biphenotypic hepatocytes” emerge in chronically injured liver. Sox9+EpCAM– Hepatocyte nuclear factor 4a-positive (HNF4α+) biphenotypic cells showing hepatocytic morphology appeared near EpCAM+ ductular structures in the livers of 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC) containing diet fed mice (Fig. 3). When MHs are labeled by LacZ expression in Mx1-Cre/ROSA mice, LacZ+Sox9+ cells emerge near ductular structures, indicating they derive from MHs. Sox9+EpCAM– cells adjacent to expanding ducts likely further convert into ductular cells, the incident is rare. Sox9+EpCAM– cells are isolated as GFP+EpCAM– cells from DDC-injured livers of Sox9–EGFP mice. Sox9+EpCAM– cells proliferate and can differentiate to functional hepatocytes in vitro and in vivo.
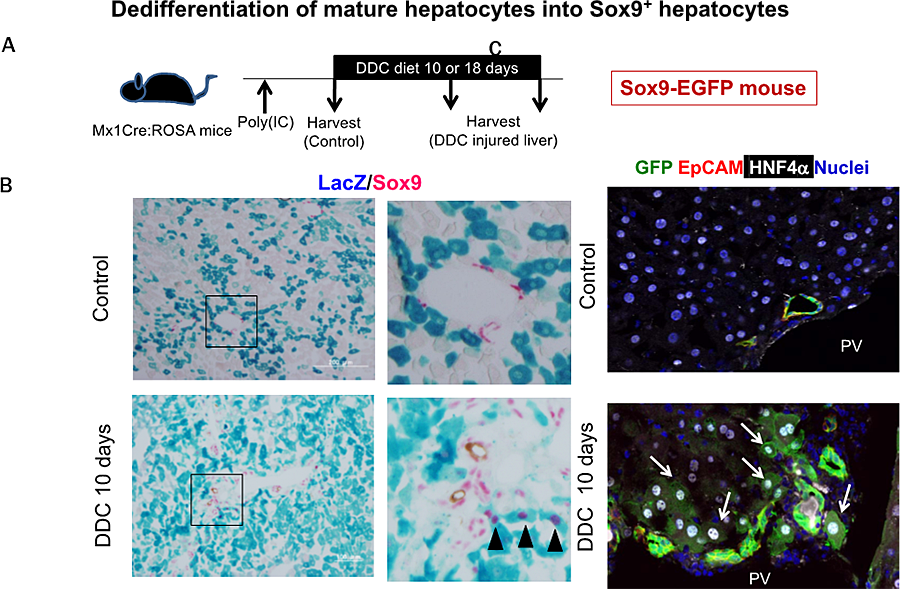 Figure 3 SOX9(+) biphenotypic hepatocytes emerge in chronically injured liver.
Figure 3 SOX9(+) biphenotypic hepatocytes emerge in chronically injured liver.
- Hepatocytes are labeled with LacZ expression in Mx1Cre mice by administration of poly(IC) before DDC-feeding.
- MHs near the portal vein dedifferentiate into SOX9(+) hepatocytes in mice fed with DDC-diet for 10 days (black arrowheads).
- In SOX9-EGFP mice, GFP(+) duct structure is expanded by DDC-injury. In addition, GFP(+) hepatocytes are observed next to the expanded ducts (white arrows).
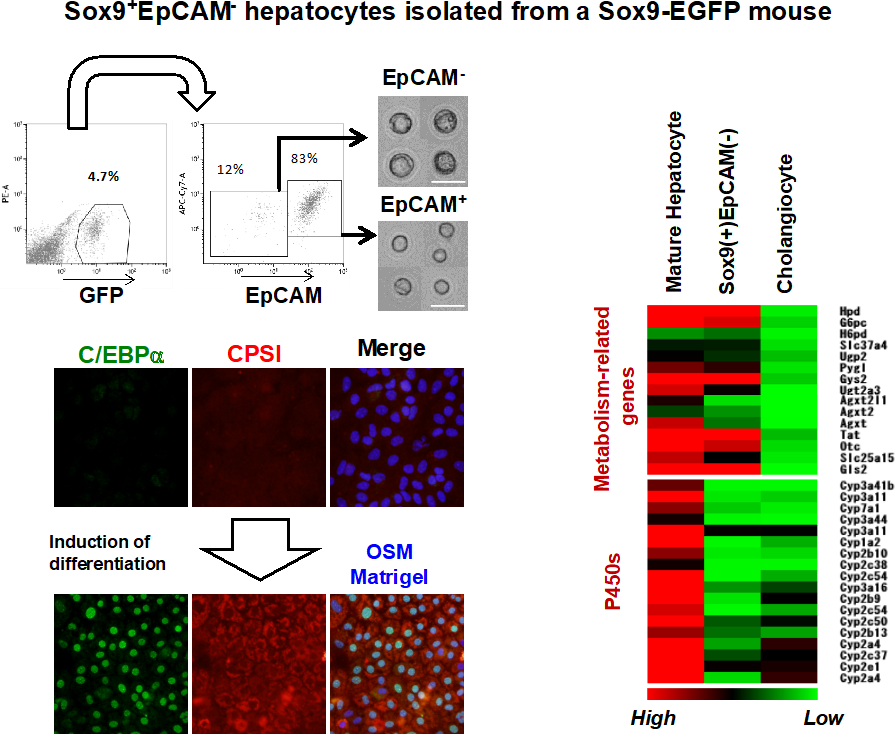 Figure 4 SOX9(+) hepatocytes show characteristics as hepatocyte progenitors.
Figure 4 SOX9(+) hepatocytes show characteristics as hepatocyte progenitors.
- SOX9(+) hepatocytes are isolated as GFP(+)EpCAM(-) cells from liver of mice fed with DDC-diet.
- SOX9(+) hepatocytes express genes related to metabolic reactions at the comparable level to MHs, whereas they lose expression of cytochrome P450s.
- SOX9(+) hepatocytes re-differentiate to mature hepatocytes positive for C/EBPα and CPSI in the culture with OSM and Matrigel.
In contrast, Sox9+EpCAM– cells form cysts with a small central lumen in collagen gels containing Matrigel® without expressing EpCAM, indicating that those cells do not acquire the potential to differentiate into cholangiocytes (Fig. 4). Considering that part of Sox9+ cells surround luminal spaces in DDC injured liver while they express HNF4α, Sox9+EpCAM– biphenotypic hepatocytes provide luminal space near expanded ductular structures to prevent deterioration of the injuries but barely convert into cholangiocytes (6,7).
- Tanimizu N, Mitaka T. Epithelial morphogenesis during liver development. Cold Spring Harb Perspect Biol, 2017; 9(8): pii: a027862
- Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM-) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem, 2014 289(11): 7589-7598
- Tanimizu N, Kobayashi S, Ichinohe N, Mitaka T. Downregulation of miR122 by grainyhead like-2 restricts the hepatocytic differentiation potential of adult liver progenitor cells. Development, (2014) 141(23): 4448-4456
- Tanimizu N, Ichinohe N, Ishii M, Kino J, Mizuguchi T, Hirata K, Mitaka T. Liver progenitors isolated from adult healthy mouse liver efficiently differentiate to functional hepatocytes in vitro and repopulate liver tissue. Stem Cells, 2016; 34(12): 2889-2901
- Tanimizu N, Ichinohe N, Yamamoto M, Akiyama H, Nishikawa Y, Mitaka T. Progressive induction of liver progenitor cells in chronically injured liver. Sci Rep, 2017; 7: 39990