3. Cell transplantation for the treatment of liver disease
Cell-based therapies have been considered as an alternative to liver transplantation for the treatment of potentially fatal liver diseases. There are mainly 3 purposes for cell transplantation; one is to replace damaged hepatocytes with fresh ones, another is to activate intrinsic liver stem/progenitor cells (LPCs), and the other is to revive the damaged cells in the recipient livers.
Thy1 is well known as a specific marker of oval cells that are one of the hepatic stem cells. Previously, we demonstrated that Thy1+ cells isolated from D-galactosamine (GalN)-treated rat livers were heterogeneous population and that some cells were bipotential hepatic stem cells with the ability to differentiate into hepatocytes through CD44+ SHs and cholangiocytes (7). Recently, we found that the Thy1+ cells have a potential to activate the intrinsic LPCs. When the Thy1+ cells were transplanted into retrorsine/partial hepatectomy (Ret/PH)-treated rat livers, the recipient’s liver rapidly regenerated and its growth continued for about 1 month (Fig. 1), resulting in liver enlargement.
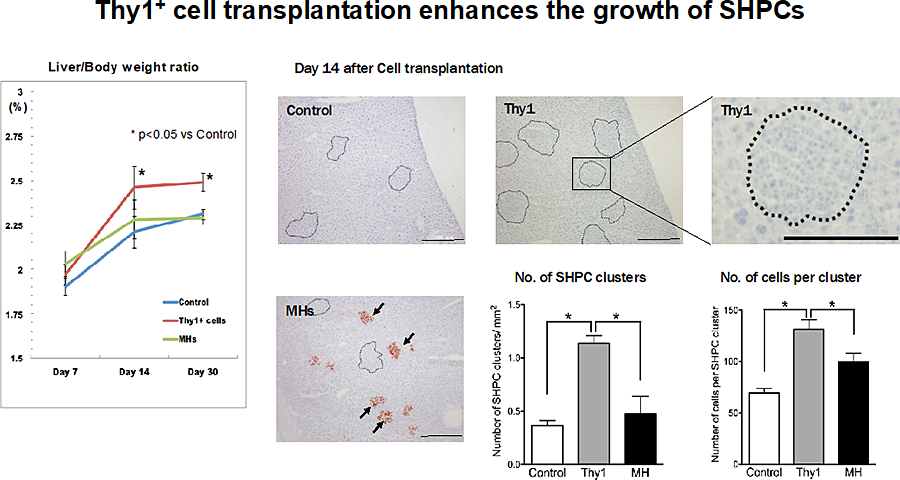
Figure 1. Emergence of SHPC clusters in retrorsine/partial hepatectomy (Ret/PH) model rat livers. Liver/body weight ratio of Control, Thy1, and MH rats were plotted. The liver/body weight ratio of Thy1 rats were significantly larger than those of Control rats at day 14 and 30. The emergence of SHPCs was investigated in Ret/PH model rat livers 14 days post-PH. Pictures shows the enzyme-histochemical analysis of DPPIV and hematoxylin staining. SHPC clusters are encircled by dotted line. DPPIV+ donor cells were engrafted and formed foci after MH transplantation (arrows). Scale bars, 500 μm. The number of SHPC clusters per area and cells per cluster were measured. Transplantation of Thy1+ cells remarkably induced the emergence of SHPCs.
Histological analysis revealed that the hepatomegaly was due to the expansion of small hepatocyte-like progenitor cells (SHPCs). We clarified the mechanism of SHPCs’ expansion (8). Transplanted Thy1+ cells secrete extracellular vesicles (EVs), which were incorporated into resident SECs, Kupffer cells and SHPCs, and then induced the secretion of interleukin 17 (IL17) B, IL25 and IL17RB, respectively (Fig. 2).
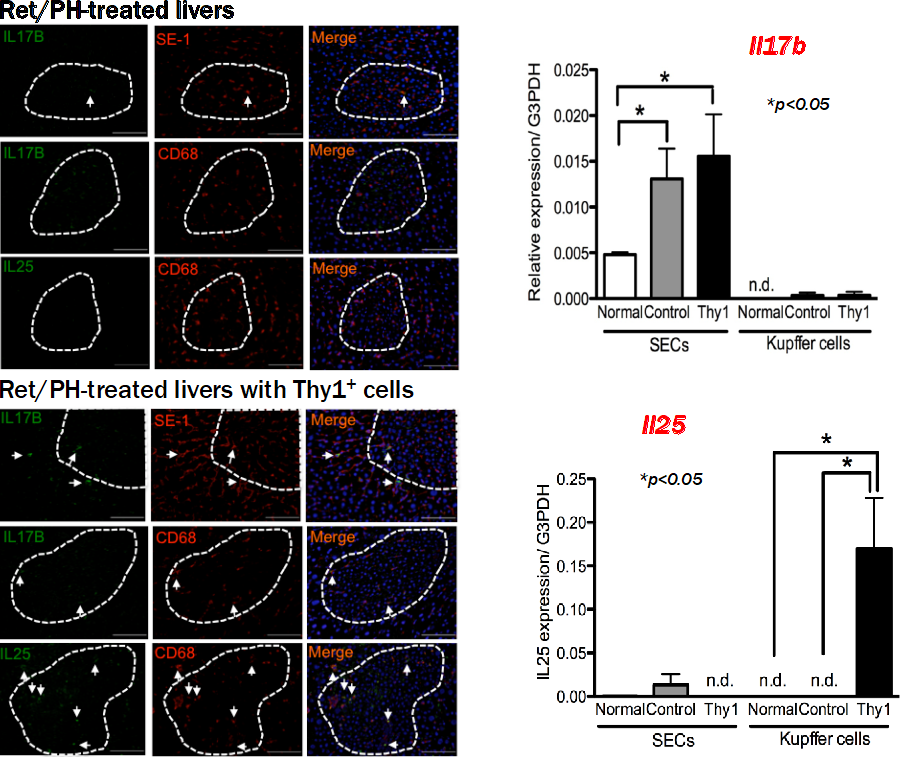
Figure 2. Localization of IL17B and IL25 proteins in SHPCs of Ret/PH model rat livers with Thy1+ cell transplantation. Double immunohistochemistry for IL17B/IL25 and markers of SECs (SE-1) and Kupffer cells (CD68) was performed using Ret/PH model rat livers with (Lower) and without (Upper) Thy1+ cell transplantation 14 days post-PH. The areas of SHPC clusters are surrounded by dotted lines. Arrows indicate double-positive cells. Scale bars, 100 μm. All images are at the same magnification. Induction of IL17b and IL25 expression in SECs and Kupffer cells by realtime-PCR. Gene expression of IL17b (upper) and IL25 (lower) was investigated in SECs and Kupffer cells isolated from livers of normal rats (Normal) and Ret/partial hepatectomy model rat livers with (Thy1) and without Thy1+ cell transplantation (Control) 14 days post-PH. Asterisk indicates statistically significant differences; p< 0.05. Abbreviations: n.d., not determined; SECs, sinusoidal endothelial cells.
Administration of EVs separated from cultured Thy1+ cells also enhanced the expansion of SHPCs (Fig. 3). The interaction of Thy1+ cell transplantation and liver regeneration is summarized in Figure 4.
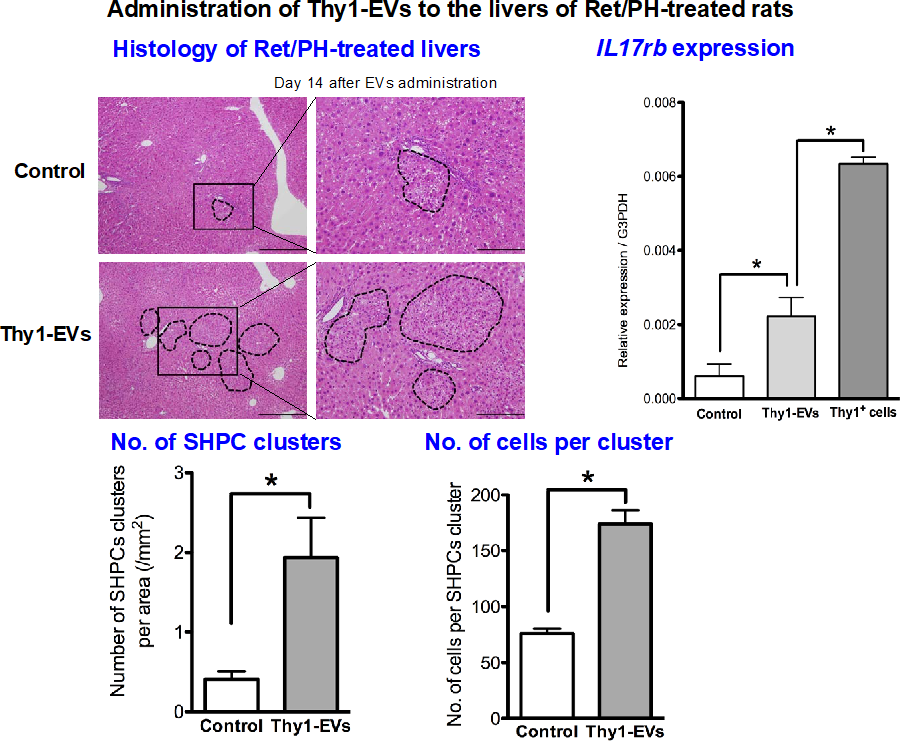
Figure 3. Administration of Thy1-EVs to Ret/partial hepatectomy model rat livers. EVs derived from the conditioned medium of Thy1+ cells for 48 hours were administered via a branch of the portal vein, and livers were examined at 14 days post-administration. Images show H-E staining of a liver section. Many SHPC clusters were detected in livers with Thy1- EVs. Right pictures represent enlargements of the squared areas in left images, respectively. Scale bars, 500 μm and 200 μm. IL17rb expression in SHPCs of livers receiving Thy1+ cell transplantation was confirmed by realtime-PCR. The number of SHPC clusters per area and the number of cells per cluster are shown. Asterisks indicate statistically significant differences; p< 0.05.
Abbreviations: EVs, extracellular vesicles; SHPCs, small hepatocyte-like progenitor cells.
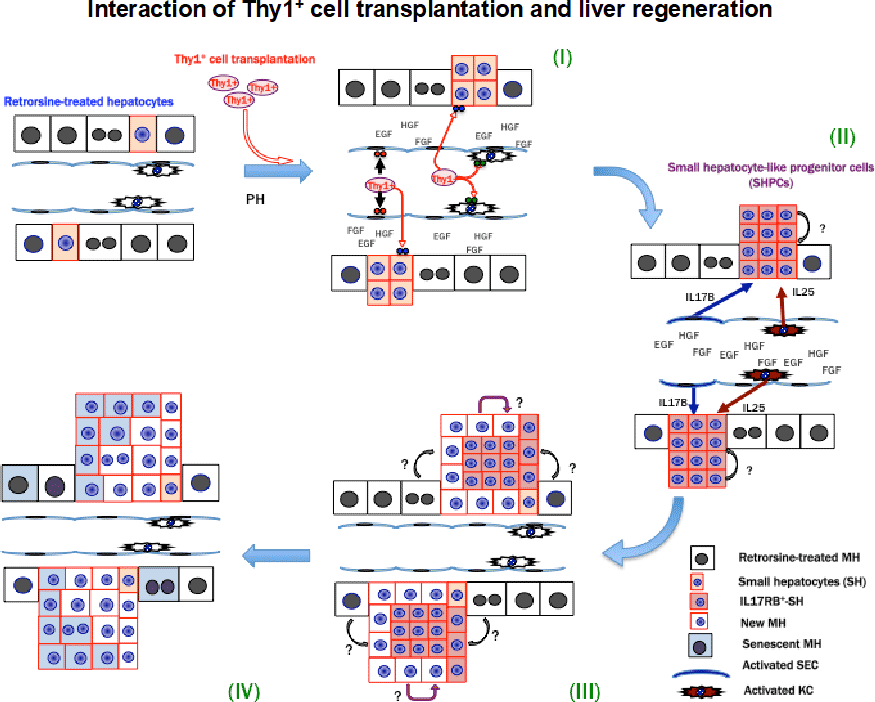
Figure 4. Summary of the interaction of Thy1+ cell transplantation and liver regeneration.
- Transplanted Thy1+ cells secrete extracellular vesicles (EVs), which were incorporated into resident SECs, Kupffer cells and SHPCs.
- Thy1-EVs induced the secretion of interleukin 17 (IL17) B, IL25 and IL17RB in SECs, Kupffer cells and SHPCs, respectively.
- IL17RB signaling stimulated the growth of SHPCs.
- Most expanded SHPCs entered cellular senescence, and the enlarged liver returned to its normal size without losing hepatic functions.
Many unsolved questions remain;
- Which factors in EVs induce IL17RB, IL17B, and IL25 in SHPCs, SECs, and Kupffer cells, respectively?
- Why can the same EVs differentially react with each cell to result in distinct reactions of each cell?
- Do other cells exist that possess the capacity to enhance intrinsic hepatic progenitor cells other than Thy1+ cells derived from GalN-induced liver injury?
Further experiments are accordingly needed to clarify these issues.
Liver failure after liver resection for patients with cirrhosis is a critical problem, and no effective therapy other than liver transplantation is available at present. To resolve this problem, we examined whether MH transplantation could prevent the post-standard liver resection mortality rate of rats with non-alcoholic steatohepatitis (NASH)-related cirrhosis. Rats were fed with a choline-deficient l-amino acid-defined diet for 12 weeks and MHs were transplanted at 24 hours before PH. MH transplantation improves the survival of the recipient rats. Thus, preoperative MH transplantation might improve the survival of rats with NASH-related cirrhosis after PH (9).
- Kon J, Ichinohe N, Ooe H et al. Thy1- positive cells have bipotential ability to differentiate into hepatocytes and biliary epithelial cells in galactosamine-induced rat liver regeneration. Am J Pathol 2009; 175: 2362–2371.
- Ichinohe N, Ishii M, Tanimizu N, Kon J, Yoshioka Y, Ochiya T, Mizuguchi T, Hirata K, Mitaka T. Transplantation of Thy1+ cells accelerates liver regeneration by enhancing the growth of small hepatocyte-like progenitor cells via IL17RB signaling. Stem Cells, 2017; 35(4): 920-931
- Nakamura Y, Mizuguchi T, Tanimizu N, Ooe H, Ichinohe N, Hirata K, Mitaka T. Preoperative hepatocyte transplantation prevents cirrhotic rats from receiving the fatal damage by a liver resection. Cell Transplant, 2014: 23(10): 1243-1254